|
|
|
|
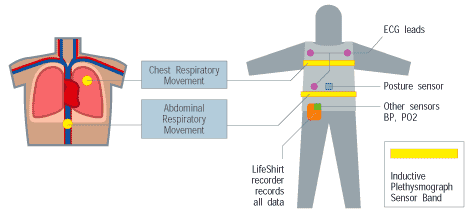
The data collection component of VivoMetrics LifeShirt System is a sleeveless undergarment that functions as a multichannel cardiopulmonary digital recorder. The shirt is made of hand-washable, reusable stretch-material into which are sewn an array of physiologic sensors to monitor 30+ vital signs. The individual being monitored can self-report symptoms, activities and medications into the PDA which then become part of the digital data stream. Any peripheral diagnostic device with digital output may be plugged into the serial port and its (their) measurements also become part of the digital data stream, e.g. pulse oximeter blood pressure, temperature, weight, etc. The data is stored on a module containing a data card that is incorporated into a customized Handspring worn on the patient’s belt or carried in a pocket. How it works
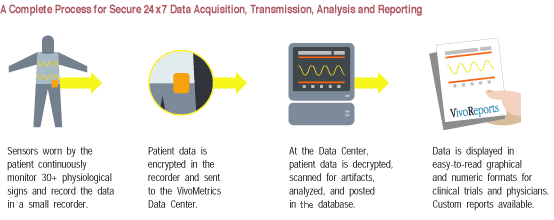
Periodically the patient will upload the data, via the Internet to the VivoMetrics' secure data center. The data center is staffed by technicians and physicians who review the quality of information and prepare it for subsequent distribution to clinical investigators or the pharmaceutical company conducting the research. Ultimately, the company hopes to take its monitoring system a step further, and introduce a wireless version that will transmit the data captured directly from the patient wearing the LifeShirt to VivoMetrics data center.
RespiEvents, the software that powers the monitoring system was developed by Non-Invasive Monitoring Systems, Inc. (NIMS). NIMS received FDA 510K clearance for expanded indication of its RespiEvents monitoring software in August 2000. This expanded indication allows for data currently monitored in hospitals to be gathered in an ambulatory mode. LifeShirt is currently not approved by the FDA, however company executives point out that the technology imbedded in LifeShirt has been in constant use in the clinical environment for many years, and hope to receive FDA clearance in the near future. Editor PDA cortex
|
|||||||